Description
Specifications of Janteng-Cord blood bag.
◆ CPDA (Citrate, Phosphate, Dextrose, Adenine) or CPD (Citrate, Phosphate, Dextrose ) ◆ 150 ml Primary bag containing anticoagulant 21 CPDA-1 or CPD solution with twin ultra thin walled sharp 16G needle, ◆ Made of high quality medical DEHP PVC sheet material which maintains aerobic metabolism ◆ Sterilized by steam in high pressure can assure products non-pyrogen and toxin-free.NEEDLE
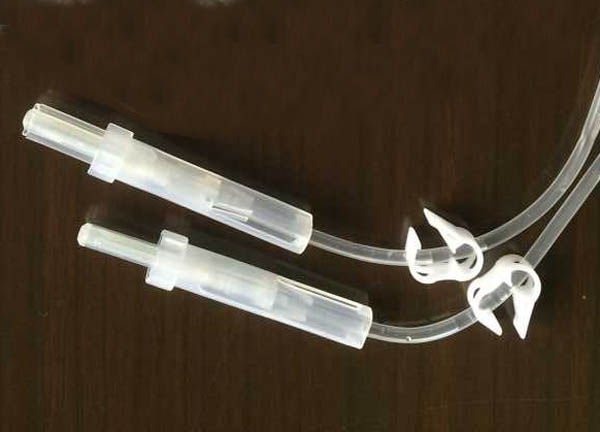
◆ Covered with protective plastic ◆ 16-G ultra thin walled sharp needles ◆ The needle bevel (< 0.5 cm) is oval split to maximise easy insertion into a donor arm. ◆ Oval shape allows sufficient needle point that allows sharp entry into a vein. ecifications of needle Pilot tube ◆ Pilot tube length is 90-120cm( Optional choice as per requirement) ◆ Each tube imprinted with a unique pilot tube number for batch tracing ◆ No air bubbles in the fluid path ◆ Made of flexible and sealable material

Medical PVC bag design
◆ Round shaped with round edges, no sharp corners ◆ Made of medical grade PVC materials that provide high ventilation with CE certification whilst maintaining in-vivo conditions ◆ Leak proof design,no risk of contamination and air embolism [ closed system ] with all leak proof seals. ◆ Simple bend of the connector tube allows blood components to be transferred smoothly into the satellite bags ◆ Flow clamps in tube ( 1~ 4 clamps as per requirement) ◆ Each bag have hanger slits on every sides for use during collection, processing and transfusion ◆ Optional sampling collection pouch and blood collection tube is attached or not as per requirement. ◆ Conventional or T&B (Top & Bottom)designing systems is also available as per requirementSpecifications of Label design
◆ None peel-off even if soaked overnight in water◆ Heat sealed labels ◆ Clearly marked with “sterile, non-pyrogenic and non-toxic” ◆ Clearly indicating volume and constituents of anti-coagulant ◆ Clearly indicating volume size of blood capacity (450ml) ◆ Clearly indicating manufacturing date, expiry date and batch(Lot)No. ◆ Clearly indicating manufacturer’s and exporter's name and address. Optional indicating local distributor or importer's name and address details.◆ Label provides sections for recording donor number, blood group and Rh factor, Serology test results and signature of attendant.
Internationally recognized quality Standards
◆ The blood bag sets are approved as per GB, CE(TUV), ISO13485( TUV), ISO3826, GMP (CFDA)
PACKAGING ◆ Protective dual packaging [individual and aluminum] eliminating microbial contamination on surface
maintaining thecontents of the bag.
◆ Easy to handle. ANTICOAGULANT of blood bags
Each 100 ml CPDA-1 [U.S.P] contains: - Citric Acid (anhydrous)……………………………………….......… 0.299g - Sodium Citrate (dehydrate)……………………………….......…..2.63g - Monobasic Sodium Phosphate (monohydrate)…......…..0.222g - Dextrose (monohydrate) ……………………………….........……… 3.19g - Adenine ……………………………………………………………..............…..0.0275g Water for injection, sufficient quantity to make…………100ml • Clear And colorless • No discoloration on storage at roomtemperature • Manufacturer to provide anticoagulant qualitycheck certificate.RESISRANCE TO DISTORTION
• When filled to normal capacity the bag is able to with stand accelerate of 500g for 30 mins at temperature 40 to 24 ℃
without becoming permanently distorted or rupture.
• bags are able to with stand temperature up – 80℃ without breakage.
The blood bag manufacturing site has been engaged in the manufacturing since 1986, it has over
200 well trained workers and a strong professional technical and quality team.
With modern and strict management, we have became a reliable brand supplier for transfusion system in world transfusion industry.
All the products have passed strict CE/MDD/,MDR (TUV) & GMP authentication system .
Tag: blood bag,single blood bag, blood collection, blood transfusion, transfusion bag, double pack
of blood bag, CPDA blood bag
Tag: blood bag, double blood bag, blood collection, blood transfusion, transfusion bag.◆ Tamper proof needle with an indicator that allows one to instantly confirm that the needle
protector has not been removed