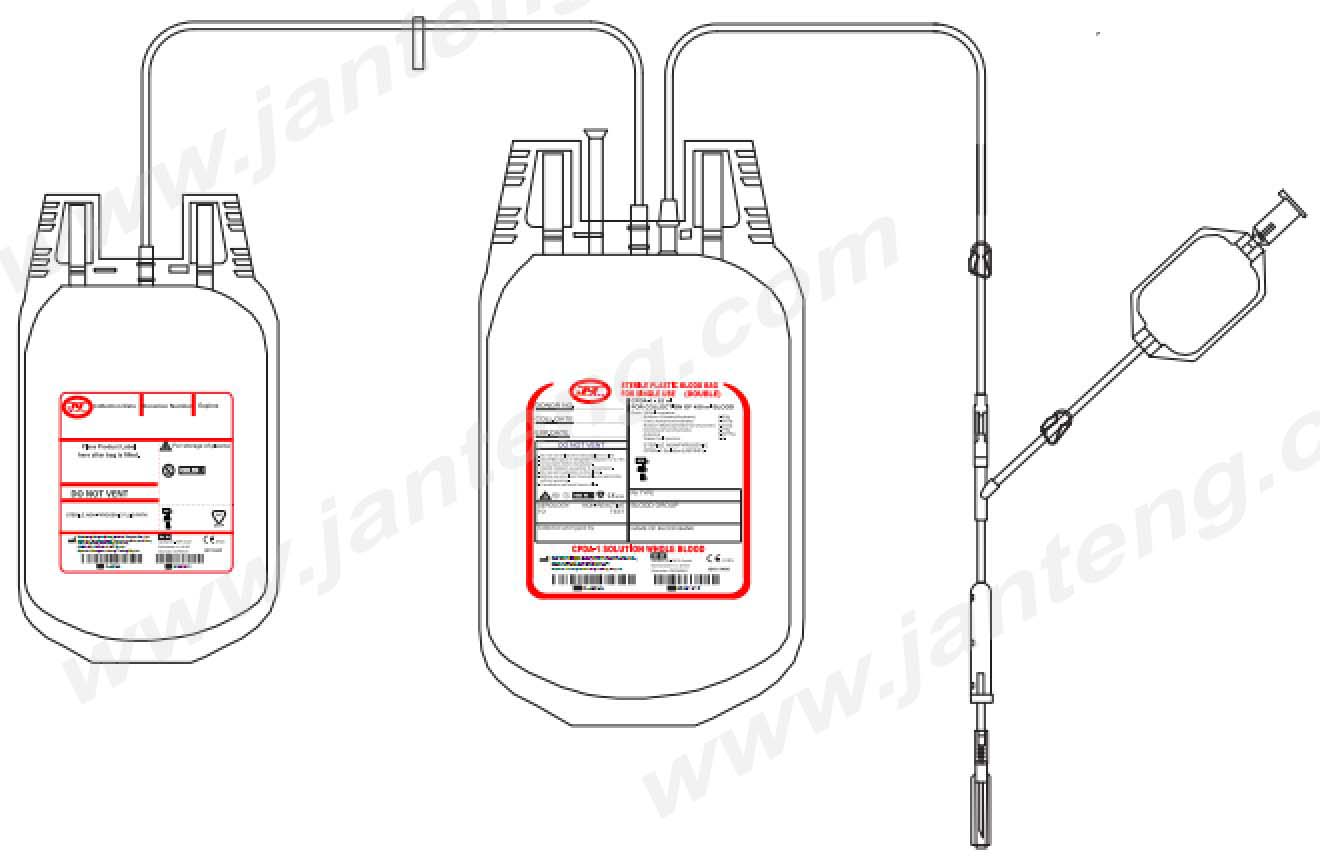
Description
General Description of Double blood transfusion system
• Sterile, pyrogen free, non toxic , ensure external sterility of the bags,
• Slit present at the bottom of the bag is satisfactory to hang the blood bag during transfusion.
• The plastic blood bag has a shelf life of minimum 2 years.
• Packing of goods: Individual blood bags are packed in a plastic pack and a maximum of 5 bags is packed in aluminum foil pack. These foil packs are packed in the corrugated boxes which indicates clearly and legibly the manufacture name,product name, batch number, quantity, manufacturing and expiry date, gross and net
weight and consignee’s name address.
• Storage of human blood and collection of blood volume is 250ml, 350ml, 450ml, 500ml.
| Code | Anticoagulant | Size | Needle | Rem |
| D250C | CPDA-1 35ml | 250ml | 16G needle | Optional NP, SP, CT OEM/PBL service accepted |
| D350C | CPDA-1 49ml | 350ml | ||
| D450C | CPDA-1 63ml | 450ml | ||
| D500C | CPDA-1 70ml | 500m |
DESIGN ofDouble blood transfusion pack
• No risk of contamination and airembolism [ closed system ] with all leak proof seals.
• Slit on both sides of the bags are enough to accommodate 5-10 ml volume test tubes.
• The capacity of the bag is enough to prevent any rupture of the bag from the seam when it is filled to the requisite volume of blood.
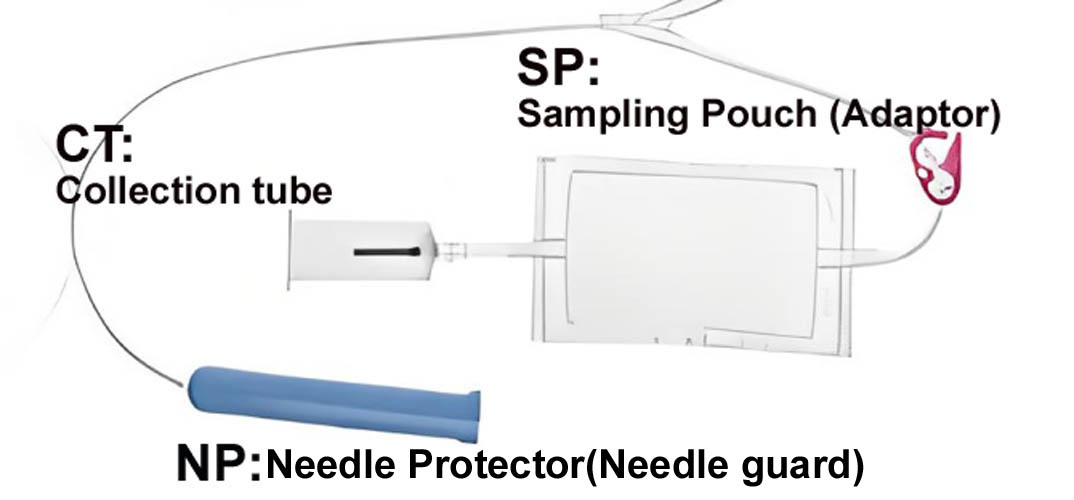
TUBING of Double blood transfusion pack
• Flexible and non kinking
• Non sticking
• Transparent
• Leak proof
• Tubing has same ID/segment number as that on the bag
• The tubes has multiple printed ID/segment numbers

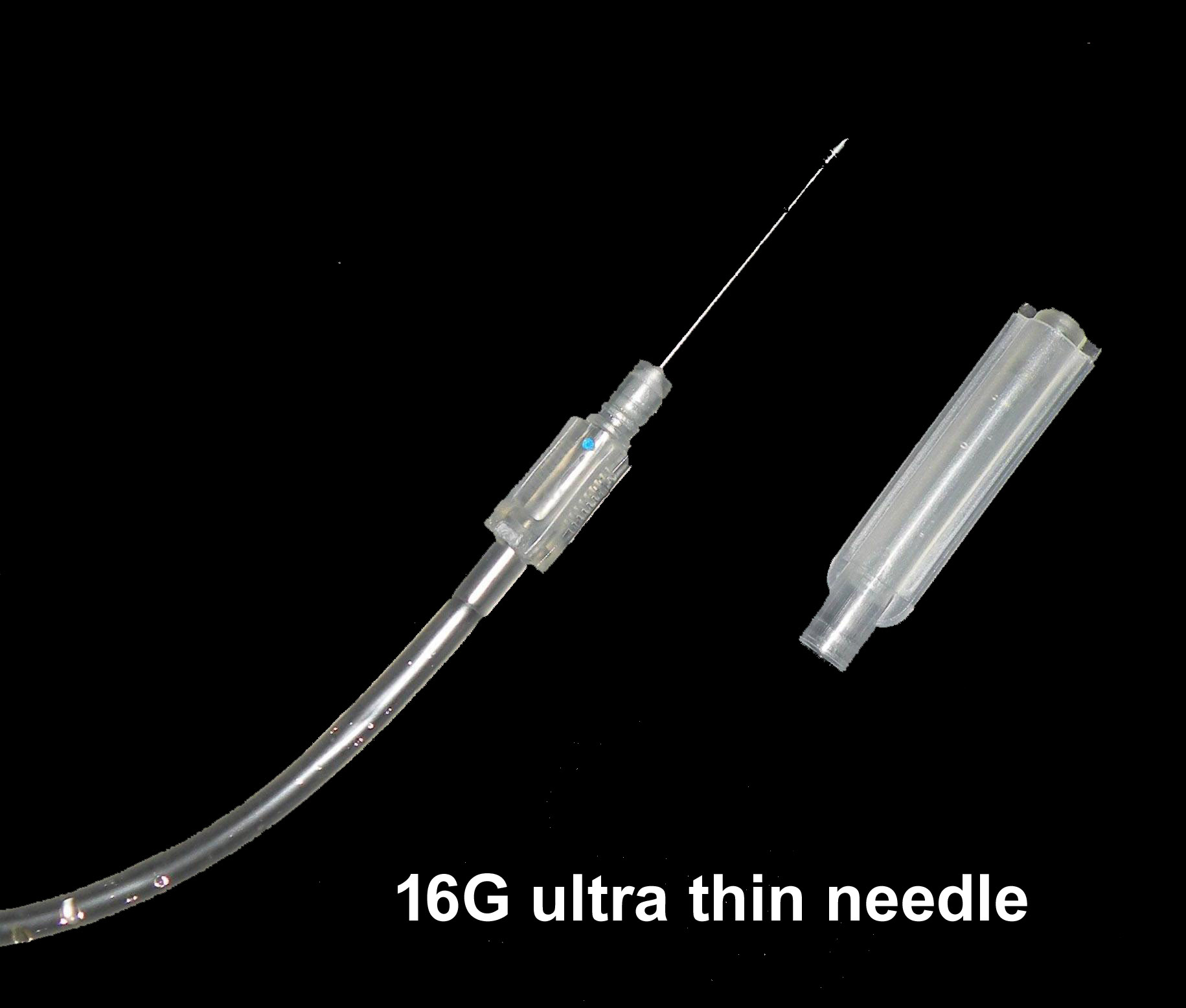
NEEDLE of Double blood transfusion pack
• 16 gauge short bevel angled
• Siliconized for maximum donor comfort
• Rust proof
• Tightly fixed with hub covered with sterile guard
• Hermetically Sealed
EXTERNAL PORT
• Tamper proof and shouldn’t be recapped
• Easily accessible
PACKAGING
• Protective dual packaging [individual and aluminum] eliminating microbial contamination on surface maintaining
the contents of the bag.
• Easy to handle.
ANTICOAGULANT of Double blood transfusion pack
Each 100 ml CPDA-1 [U.S.P] contains:
- Citric Acid (anhydrous)……………………………………….......… 0.299g
- Sodium Citrate (dehydrate)……………………………….......…..2.63g
- Monobasic Sodium Phosphate (monohydrate)…......…..0.222g
- Dextrose (monohydrate) ……………………………….........……… 3.19g
- Adenine ……………………………………………………………..............…..0.0275g
Water for injection, sufficient quantity to make……………100ml
• Clear And colorless
• No discoloration on storage at room temperature
• Manufacturer to provide anticoagulant quality check certificate.
LABEL ofDouble blood transfusion pack
• Non peel off
• Heat sealed labels
• Remain attached between room temperature to80 c with a transparent adhesive
• Label has printed manufacturing and expiry date and lot number of the bag
• Expiry date 2 years from the date of sterilization
• Label provides sections for recording donor number, collection and expiration date of blood, blood group and Rh factor,Serology test results and signature of attendant.
RESISTANCE TO DISTORTION
• When filled to normal capacity the bag is able to with stand accelerate of 500g for 30 mins at temperature 40 to 24 ℃ without becoming permanently distorted or rupture.
• bags are able to with stand temperature up – 80℃ without breakage.
The blood bag manufacturing site has been engaged in the manufacturing since the 1980s. It has over 200 well trained workers and a strong professional technical and quality team. With modern and strict management, we have become a reliable brand supplier for transfusion system in world transfusion industry. All the products have passed strict CE/MDD/MDR (TUV) & GMP authentication system . Our manufacturing site has been registered and approved in more than thirty countries's MOH or health authorities/drug adminstrations.
Tag: blood bag, double blood bag, blood collection, blood transfusion, transfusion bag, plastic blood bag,human blood bag